In November of 2016, medical device maker Stryker once again recalled one of their artificial hip implants. This time, it’s the LFIT CoCr V40. The statute of limitations for this defective product may be running out, so potentially affected patients should act now.
What is it, and what is the problem?
The “LFIT Anatomic CoCr V40 Femoral Head” is a modular component used in hip replacement. The LFIT CoCr V40 is designed to be locked onto the femoral hip stem trunnion during a total hip replacement surgery.
Patients with the LFIT CoCr V40 implant are experiencing heavy metal poisoning, corrosion, and early hip failure, among other health problems. More surgery is typically the only option for recovery.
All of these complications are found in other, prior metal-on-metal hip products.
(This blog has covered the Stryker recall before, as well as similar ones. In 2012, we wrote about Stryker’s flawed “Rejuvenate” and “ABG-II” hip implants, which had a high early failure rate. In 2011, we wrote about DePuy’s recall of hip implants with an astonishing 8-in-10-patient revision rate, meaning 80% of the implants failed.)
How common is the Stryker defective hip implant?
It’s not yet clear how many patients received Stryker’s LFIT CoCr V40. However, total hip replacement is a very common surgery. 270,000 total hip replacements are performed every year in the United States according to NCBI. That number will rise to 570,000 by 2030.
Hip implants improve the quality of life of patients who suffer from osteoarthritis, hip fractures, and other hip joint trouble. But, there are problems with some of these implants, such as the Stryker LFIT CoCr V40 series.
Voluntarily recalls of these devices have become common. From 2002 to 2013, Consumers Union reported six major hip replacement component recalls from top ortho-medical device companies. The list includes Biomet, DePuy, Smith & Nephew, Stryker, Wright, and Zimmer.
Questions & Answers about Stryker’s defective LFIT CoCr V40 recall
Q: What are the symptoms of a defective hip implant?
A: Stryker notified medical providers that patients who received a defective LFIT CoCr V40 implant may experience any of these symptoms:
- loss of mobility
- pain
- inflammation
- adverse local tissue reaction
- dislocation
- joint instability
- implant loosening
- periprosthetic fracture
- leg length discrepancy
These symptoms may be indicative of serious health conditions. If you are experiencing any of these symptoms, you should contact your surgeon as soon as possible.
Q: Is there a test for a defective femoral head hip implant?
A: Blood labs and imaging studies may show a defect in your implant. High levels of Cobalt and Chromium in the blood are a sign of a defective implant. A “Metal Artifact Reduction Sequence” (MARS) MRI can show soft tissue damage around the hip implant, which is also a sign of a defective implant.
Q: How will my surgeon know if I have a defective Stryker implant?
A: Stryker sent urgent recall notices to recipient hospitals and surgeons of their recalled devices. Although the notice lists only the reference numbers of the recalled LFIT CoCr V40 lots, there may be other lots causing the same medical problems.
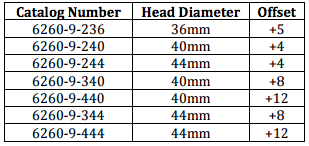
What to do if you might have a defective Stryker LFIT CoCr V40 femoral head hip implant
Step 1: Contact your surgeon
Your surgeon may ask if you are experiencing any symptoms. Stryker notified medical providers that patients who received a defective LFIT CoCr V40 implant may experience any of these symptoms:
- loss of mobility
- pain
- inflammation
- adverse local tissue reaction
- dislocation
- joint instability
- implant loosening
- periprosthetic fracture
- leg length discrepancy
These symptoms may be indicative of conditions that have serious effects on your health and could lead to a need for a revision surgery. If you are experiencing any of these symptoms, you should contact your surgeon as soon as possible.
Step 2: Obtain your medical records
The Operative Report from a hip replacement surgery should list the make and model of the device and components implanted in patients who underwent this procedure.

Our team of experienced personal injury attorneys can also help you determine if your hip replacement components are part of a recall. SUGARMAN’s attorneys represented a significant number of patients who were injured by other metal-on-metal hip implants, including the Stryker Rejuvenate and ABG II hip implants, and the DePuy ASR XL and ASR Systems.
If you have any questions regarding a Stryker LFIT CoCr V40 femoral head implant, please contact SUGARMAN principals Benjamin Zimmermann and David McCormack at 617-542-1000. You can also send us an email at .
